Interim guidance for healthcare providers and Zika virus laboratory diagnosis
Public health guidance
As of 29 February 2016, the spread of the Zika virus epidemic in the Americas and Caribbean is continuing. In the light of the current disease trend and the association with severe complications (such as adverse pregnancy outcomes or neurological complications), ECDC is proposing an algorithm for public health management of cases under investigation for Zika virus infection and an outline of the strategy for the laboratories performing Zika virus infection diagnostic tests.
This document aims to present an algorithm for deciding whom to test and provide guidance on the laboratory tests for Zika virus infection diagnosis in order to support clinical diagnostic and case reporting through surveillance among EU Member States. The information is provisional and subject to revision when new information becomes available.
This document aims to present an algorithm for deciding whom to test and provide guidance on the laboratory tests for Zika virus infection diagnosis in order to support clinical diagnostic and case reporting through surveillance among EU Member States. The information is provisional and subject to revision when new information becomes available.
Publication file
Interim guidance for healthcare providers and Zika virus laboratory diagnosis
English (293.8 KB - PDF)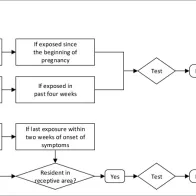
Data
The aim of this algorithm is to determine when a person who has been exposed to Zika virus needs to be tested and notified, and when vector control measures should be considered around a case.

Tools for public health
Laboratories should receive clinical and epidemiological information for establishing their investigation strategy, including date of onset of illness, travel history (date and locations), past flaviviral immunisation records and pregnancy status.
Share this page
