Laboratory tests for Zika virus diagnostic
Tools for public health
Laboratories should receive clinical and epidemiological information for establishing their investigation strategy, including date of onset of illness, travel history (date and locations), past flaviviral immunisation records and pregnancy status. More information on conducting Zika virus diagnostic is available in the ECDC Interim guidance for healthcare providers and Zika virus laboratory diagnosis, which contains an outline of the strategy for the laboratories performing Zika virus infection diagnostic tests.
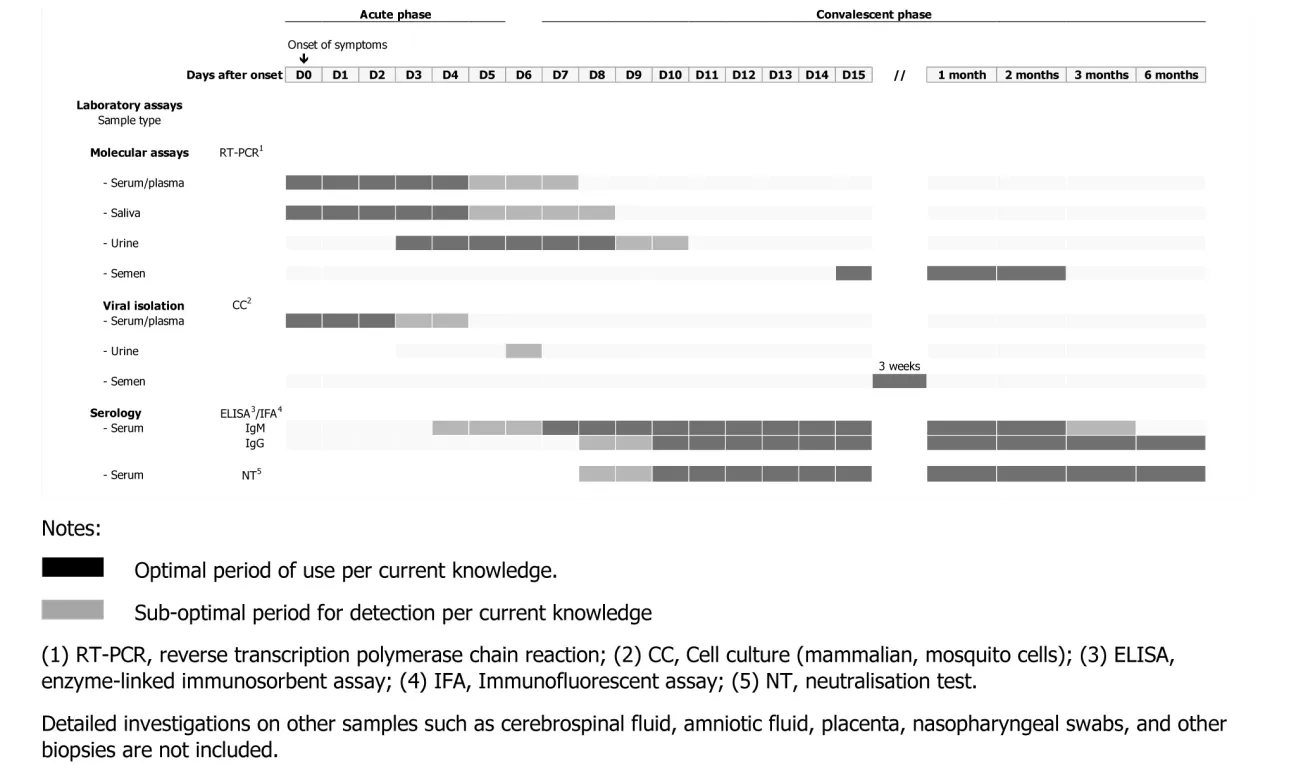
Laboratory tests for Zika virus diagnostic
English (253.8 KB - JPG)