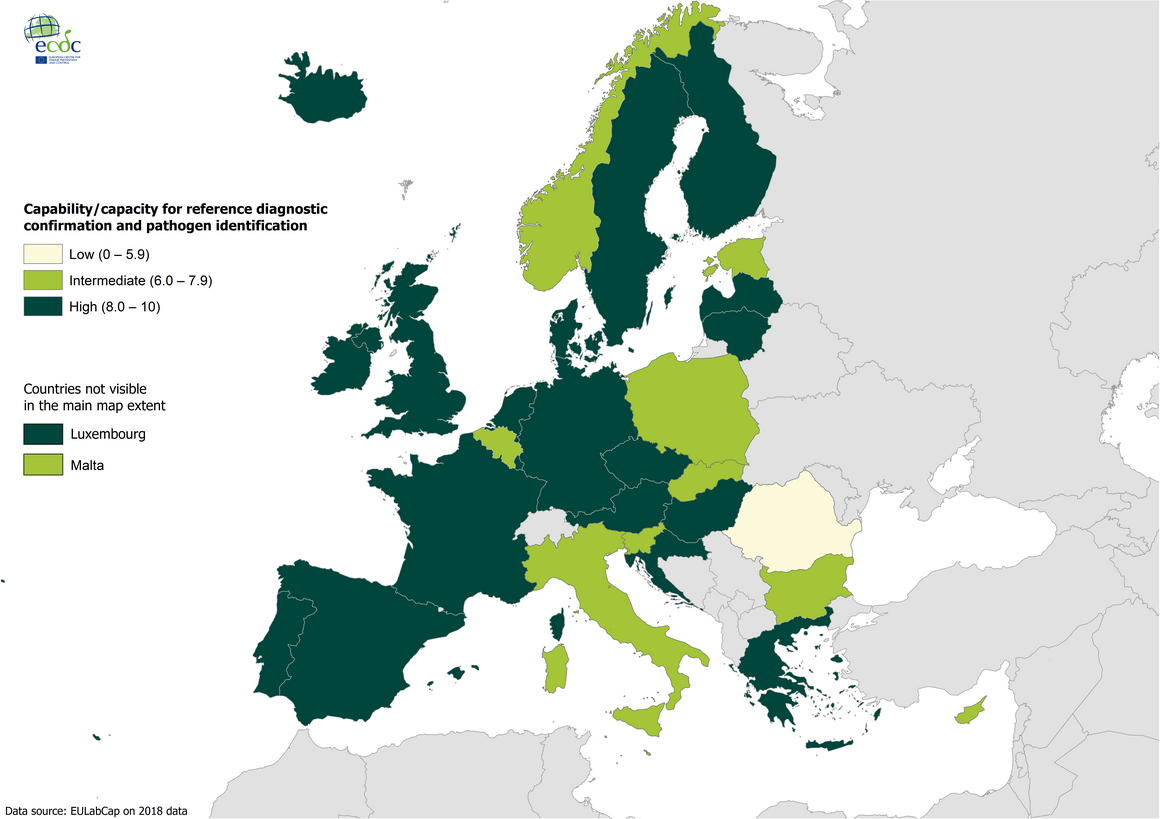
Target 2.2 - Reference diagnostic confirmation and pathogen identification 2018
Availability of national reference laboratory testing capability and capacity and a robust sample referral and reporting system to the national authorities is a prerequisite for effective surveillance and epidemic preparedness at national and EU levels in accordance with NMFP consensus.
Download
Target 2.2. Reference diagnostic confirmation and pathogen identification
Availability of national reference laboratory testing capability and capacity and a robust sample referral and reporting system to the national authorities is a prerequisite for effective surveillance and epidemic preparedness at national and EU levels in accordance with NMFP consensus.




