Public health guidance in brief on HIV, hepatitis B and C testing in the EU/EEA
This Guidance in brief is based on the comprehensive guidance document which provides the evidence base for this guidance
Executive Summary
The ECDC guidance on integrated testing of hepatitis B (HBV), hepatitis C (HCV) and HIV supports countries in the global effort to combat viral hepatitis and eliminate HIV as public health threats by 2030. At present, reaching and testing those at risk of infection with HIV, HBV or HCV is still a public health challenge across Europe.
Increasing testing coverage and uptake, especially for those most at risk, is an essential element of any strategy to eliminate HIV, HBV and HCV in the European Union and European Economic Area (EU/EEA). In order to interrupt existing transmission chains and prevent further infections, Europe needs a stronger focus on working closely with vulnerable populations. This will improve efforts to identify those who are infected but not diagnosed and link them to appropriate healthcare services.
Public health guidance in brief on HIV, hepatitis B and C testing in the EU/EEA
English (681.1 KB - PDF)Read the full report

This guidance aims to provide EU/EEA countries with an evidence-based framework to help develop, implement, monitor and evaluate their own national HBV, HCV and HIV testing guidelines and programmes.
Public health guidance on HIV, hepatitis B and C testing in the EU/EEA
English (1.8 MB - PDF)Read more on the ECDC website
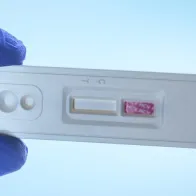
To mark European Testing Week from 23 to 30 November 2018, ECDC publishes its new Guidance on integrated viral hepatitis and HIV testing.
All updates on HIV and hepatitis B and C
Featured
Share this page






