Epidemiological update: SARS-CoV-2 and NB.1.8.1 variant assessment
Key messages
- In the European Union and the European Economic Area (EU/EEA), respiratory consultation rates are currently at baseline levels or have returned to levels observed at this time in previous seasons.
- Slow increases in SARS-CoV-2 epidemiological indicators have been observed in many EU/EEA countries in recent weeks, however levels remain low and no significant impact on secondary care or on the number of deaths (including excess mortality) has been observed to date.
- NB.1.8.1 is not expected to cause an increased risk to public health compared to other recently circulating Omicron-descendant SARS-CoV-2 variants.
- No clinical studies are currently available for COVID-19 vaccine effectiveness for NB.1.8.1, but no significant impact on vaccine effectiveness against severe disease is anticipated based on its mutation profile and early laboratory studies. NB.1.8.1 is currently circulating at low proportions in the EU/EEA but is expected to rise in the coming weeks.
- Of note, population immunity has most likely fallen following a winter with low circulation of SARS-CoV-2 which may lead to some increases not only of SARS-CoV-2 infections, but also of hospitalisations in the coming weeks, particularly among groups at risk of severe disease, such as older adults.
- Additionally, during the recent winter season, COVID-19 vaccine coverage in older adults was suboptimal in several EU/EEA countries with only one country reaching more than 80% coverage, and seven countries reaching more than 50% coverage, in people older than 80 years of age, for whom burden of severe outcomes is the highest.
- In modelling projections for June-December 2025, decreasing population immunity against SARS-CoV-2 alone is sufficient to drive a COVID-19 wave in the EU/EEA, in the absence of any changes to variant properties for transmissibility and/or severity. A peak in weekly hospital admissions is projected to be of comparable magnitude to what was observed in the summer of 2024.
- Surveillance of respiratory viruses should continue year-round and be reported without delay. This is particularly important given the absence of a clear seasonal pattern for SARS-CoV-2 transmission.
- In case of sustained increases in COVID-19 infections, risk communication activities for the public should be implemented, including tailored guidance for groups at increased risk of severe disease, healthcare workers and carers of individuals vulnerable to severe outcomes.
- It is important that members of groups at risk of severe disease, including older adults, continue to keep up to date with their COVID-19 vaccination, as per national recommendations, to protect against severe disease over time.
- Vaccination could also be considered for individual benefit, in specific populations such as the very elderly, outside the context of planned vaccination campaigns, as the epidemiological situation requires.
- Timely protection with available COVID-19 vaccines, when individual risk is considered high, should take precedence over availability of the most recently updated vaccines.
Epidemiological situation
Background
EU/EEA countries have experienced limited SARS-CoV-2 circulation in recent months with no winter epidemic [1] which may have increased the EU/EEA population’s susceptibility to SARS-CoV-2 infections.
Data in the European Respiratory Virus Surveillance Summary (ERVISS - https://erviss.org/) indicate that, following winter epidemics of influenza and respiratory syncytial virus (RSV), in recent weeks, many countries have reported increases in indicators of SARS-CoV-2 from previous low levels , with overall trends similar to those observed in summer 2024. No significant increase of severe COVID-19 (hospitalisations, admissions to intensive care units, deaths) has been observed to date. Preliminary analysis of data from two countries indicates that since winter 2023, the impact on hospital admissions during the COVID-19 epidemics has been comparable to that of seasonal influenza epidemics, with hospitalisations and deaths concentrated among older adults (mainly over 80 years of age).
Recent increases in SARS-CoV-2 infections have also been reported in some Asian countries [2]. According to WHO, test positivity for SARS-CoV-2 has been increasing since mid-February, particularly in the Eastern Mediterranean, South-East Asian and Western Pacific regions. Many of these infections were due to a new SARS-CoV-2 Omicron-descendant variant called NB.1.8.1 [3].
NB.1.8.1 variant
NB.1.8.1 is a descendent of Omicron variants which have circulated previously in the EU/EEA. Increases in SARS-CoV-2 infections have been observed in Asia in recent weeks, associated with increased detections of NB.1.8.1 in some, but not all countries [2-6]. The growth advantage of NB.1.8.1 relative to other circulating strains in these contexts has been attributed to a mix of factors, including waning immunity in the population following a period of low SARS-CoV-2 circulation, and a slight advantage in immune evasion for NB.1.8.1. However, there is currently no evidence of increased severity for NB.1.8.1 when compared to previously dominant variants in countries in Asia where it is more established.
While there are no epidemiological studies assessing transmissibility of NB.1.8.1 relative to other circulating strains, a very limited number of available laboratory studies are informative, with two studies assessing in vitro infectivity showing lower infectivity of NB.1.8.1 versus LP.8.1 and XEC, previously dominant Omicron variants in the EU/EEA [7,8].
No clinical studies are currently available assessing COVID-19 vaccine effectiveness for NB.1.8.1. However, no significant impact on vaccine effectiveness against severe disease is anticipated based on its mutation profile and early laboratory studies, including one study that confirmed the ability of serum from individuals vaccinated with a JN.1 mRNA vaccine booster to neutralise NB.1.8.1 in vitro [8].
ECDC is tracking five SARS-CoV-2 variants currently circulating in the EU/EEA, including NB.1.8.1, now classified as a variant under monitoring by both WHO and ECDC [9]. NB.1.8.1 is currently circulating at low proportions in the EU/EEA but is expected to rise in coming weeks. While this VUM classification serves to highlight the evolution of a SARS-CoV-2 variant that is outcompeting other strains, the current assessment of N.B.1.8.1 is that this variant should not cause an additional public health risk compared to other circulating SARS-CoV-2 variants.
Surveillance gaps in the EU/EEA
The number of EU/EEA countries reporting primary care surveillance data decreases in the summer months (weeks 21 to 39) compared to the traditional winter respiratory viruses surveillance season (weeks 40 to 20). Non-sentinel laboratory-based data (which comes from a mix of primary care and other sources, including hospital laboratories) is needed to fill the gaps. However, the number of countries reporting these data for SARS-CoV-2, particulalry for severe diseases, has also fallen over time. Almost half of EU/EEA countries do not undertake year-round monitoring of any indicator of severe COVID-19. This makes it challenging to assess the impact on hospitals and mortality of increased virus transmission in the summer months. The ‘ECDC/WHO operational considerations for integrated surveillance of respiratory viruses’ from 2022 [10] recommend that surveillance of respiratory viruses should continue year-round with timely, weekly reporting to ECDC. This is particularly important in the absence of an established seasonal pattern of SARS-CoV-2 transmission.
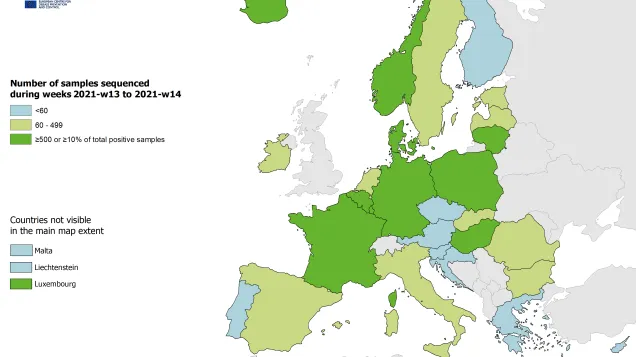
Estimates of the relative circulation of different variants in the EU/EEA in the past months have been based on data from a small number of countries sequencing at low volumes, due in part to the limited availability of specimens. ECDC/WHO recommend representative sequencing based on all detections from ILI/ARI/SARI surveillance systems. Greater precision can be achieved by also sequencing specimens from other sources. Higher sequencing volumes should be achievable given increased SARS-CoV-2 detections.
Scenario projections for summer 2025 by mathematical modelling
ECDC has conducted scenario projections for COVID-19 dynamics in the EU/EEA from June - December 2025. Three key insights were obtained from the scenario projections. First, the model suggests that population-level immunity has waned substantially since the end of 2024, due to the low-levels of SARS-CoV-2 circulation in winter 2024/25. In particular, for the six considered countries, population-level immunity is at slightly lower levels in June 2025 when compared to June 2024, i.e. slightly lower than the levels before the COVID-19 waves in 2024. Second, the scenario projections suggest that the low population-level immunity alone—in the absence of any changes to variant transmissibility and/or severity--is sufficient to drive a COVID-19 wave in the EU/EEA in summer 2025. Third, the scenario projections suggest a peak in hospital admissions of comparable magnitude to the one that occurred during summer 2024, albeit with marked variations and uncertainty across countries and scenarios.
Scenario projections were generated by a compartmental SIRS-type COVID-19 model with age-specific contact patterns and disease severity, seasonality, vaccination, and waning of naturally acquired and vaccine-induced immunity. Based on a Kalman filter approach, the model was calibrated to COVID-19 non-sentinel hospitalisation data of six countries with sufficiently high data quality in the EU/EEA, and the fitting period was one year.
There are various limitations to these results, since the model relies on several assumptions, including similar behaviour, vaccine uptake, and surveillance as in 2024. Furthermore, the model assumed that there is no new SARS-CoV-2 variant with substantially different characteristics than the currently circulating variants. Lastly, there are limitations in the quality and availability of COVID-19 hospitalisation data, which was used to calibrate the model; particularly, it is unclear to what extent the results translate to countries that did not report COVID-19 hospitalisation data with sufficient quality.
Vaccination coverage, effectiveness
The most recent COVID-19 vaccination campaigns were implemented widely in EU/EEA countries in autumn 2024. The vaccination coverage data for this period show that seven countries (out of 21 reporting countries) reported a vaccination coverage above 50% in individuals above the age of 80 years and eight countries reported a vaccination coverage above 30% in individuals above the age of 60 years [11].
The most recent vaccine effectiveness data related to JN.1 and KP.2 adapted vaccines in the context of late 2024 and the first months of 2025, indicates that current COVID-19 vaccines provide protection against disease, especially against severe disease and deaths. This has been shown through ECDC studies [12] on vaccine effectiveness under the Vaccine Monitoring Platform (VMP) [13] - Vaccine Effectiveness, Burden and Impact Studies (VEBIS) [14] and confirmed by studies outside the EU/EEA [15-18]. However, COVID-19 vaccine protection wanes over time, more rapidly for infection and moderate disease, due to the evolution of the virus and the immune system [19].
At the time of the publication of this COVID-19 epidemiological update, no real world data is available on the vaccine effectiveness against the variant NB.1.8.1.
Recommendations
- Surveillance of SARS-CoV-2 should continue in summer 2025 and be reported without delay, in line with ECDC/WHO recommendations for year-round integrated surveillance of respiratory viruses [10].
- Representative sequencing based on all detections from ILI/ARI/SARI surveillance systems should be performed. Greater precision can be achieved by sequencing specimens from other sources. Higher sequencing volumes should be achievable given increased SARS-CoV-2 detections.
- It is important that groups at high risk for severe disease, including older adults, continue to keep up to date with their COVID-19 vaccination, as per national recommendations, to protect against severe disease over time.
- Combined autumn/winter vaccination campaigns against COVID-19 and influenza are considered efficient strategies in terms of administration, logistics, costs and therefore overall benefit for the population. In addition to planned vaccination campaigns, to maximise individual protection in specific individuals, consideration could also be given to offering further COVID-19 vaccination doses during other times of the year that target the groups very vulnerable to severe disease such as those 80 years of age and above, and individuals with certain underlying conditions, irrespective of age, depending on time since the last dose (with each dose administered with at least a three month interval after the most recent dose of a COVID-19 vaccine) and the epidemiological situation.
- Timely protection with available COVID-19 vaccines, when individual risk is considered high, should take precedence over waiting for availability of the most recently updated vaccines.
- In case of sustained increases in COVID-19 cases, risk communication activities for the public should be implemented, including tailored guidance for groups at higher risk of severe disease, healthcare workers and carers of groups vulnerable to severe outcomes. Key recommendations include staying home when ill, respiratory etiquette and good hand hygiene, appropriate ventilation of indoor spaces, and promotion of appropriate public health and social measures (PHSM) (also called non-pharmaceutical interventions (NPIs)). People with high risk for severe disease (as well as their carers and close contacts) should consider using a face mask when in crowded public spaces [20].
Consulted ECDC experts
Erik Alm, Sabrina Bacci, Eva Bons, Nick Bundle, Edoardo Colzani, Luisa Hallmaier-Wacker, Phyo Myint, Priyanka Nannapaneni, Rene Niehus, Kate Olsson, Ajibola Omokanye, Bastian Prasse, Olov Svartstrom.
References
1. European Centre for Disease Prevention and Control. European Respiratory Virus Surveillance Summary (ERVISS). Available at: https://erviss.org/
2. World Health Organization. COVID-19 Global Situation - 28 May 2025. 2025. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2025-DON572
3. World Health Organization. WHO TAG-VE Risk Evaluation for SARS-CoV-2 Variant Under Monitoring: NB.1.8.1. 2025. Available at: https://cdn.who.int/media/docs/default-source/documents/epp/tracking-sars-cov-2/23052025_nb.1.8.1_ire.pdf
4. Chinese Center for Disease Control and Prevention. Novel Coronavirus Infection Situation in China (May 2025). 2025 Available at: https://www.chinacdc.cn/jksj/xgbdyq/202506/t20250605_307356.html
5. Hong Kong Centre for Health Protection. COVID-19 & Flu Express (Volume 3), Number 23 (Week 23, 2025). Available at: https://www.chp.gov.hk/files/pdf/covid_flux_week23_12_6_2025_eng.pdf
6. Singapore Ministry of Health. Press release: Update on COVID-19 situation (13/05/2025). Available at: https://www.moh.gov.sg/newsroom/update-on-covid-19-situation-
7. Guo C, Yu Y, Liu J, Jian F, Yang S, Song W, et al. Antigenic and virological characteristics of SARS-CoV-2 variants BA.3.2, XFG, and NB.1.8.1. Lancet Infect Dis. 2025 Jun 5 Available at: https://www.ncbi.nlm.nih.gov/pubmed/40484018
8. Uriu K, Okumura K, Uwamino Y, Chen L, Tolentino JE, Asakura H, et al. Virological characteristics of the SARS-CoV-2 NB.1.8.1 variant. Lancet Infect Dis. 2025 Jun 6 Available at: https://www.ncbi.nlm.nih.gov/pubmed/40489985
9. European Centre for Disease Prevention and Control. SARS-CoV-2 variants of concern as of 28 May 2025. 2025. Available at: https://www.ecdc.europa.eu/en/covid-19/variants-concern
10. European Centre for Disease Prevention and Control. Operational considerations for respiratory virus surveillance in Europe. 2022. Available at: https://www.ecdc.europa.eu/en/publications-data/operational-considerations-respiratory-virus-surveillance-europe
11. European Centre for Disease Prevention and Control. COVID-19 vaccination coverage. Available at: https://www.ecdc.europa.eu/en/covid-19/prevention-and-control/vaccines#vaccination-coverage
12. Laniece Delaunay C, Verdasca N, Monge S, Domegan L, Seve N, Buda S, et al. COVID-19 Vaccine Effectiveness Against Medically Attended Symptomatic SARS-CoV-2 Infection Among Target Groups in Europe, October 2024-January 2025, VEBIS Primary Care Network. Influenza Other Respir Viruses. 2025 May;19(5):e70120. Available at: https://www.ncbi.nlm.nih.gov/pubmed/40395132
13. European Centre for Disease Prevention and Control. Vaccine Monitoring Platform. Available at: https://www.ecdc.europa.eu/en/about-ecdc/partners-and-networks/eu-institutions-and-agencies/vaccine-monitoring-platform
14. European Centre for Disease Prevention and Control. Vaccine Effectiveness, Burden and Impact Studies (VEBIS). Available at: https://www.ecdc.europa.eu/en/infectious-disease-topics/related-public-health-topics/immunisation-and-vaccines/vaccine-0
15. Hansen CH, Lassauniere R, Rasmussen M, Moustsen-Helms IR, Valentiner-Branth P. Effectiveness of the BNT162b2 and mRNA-1273 JN.1-adapted Vaccines Against COVID-19-associated Hospitalisation and Death: a Danish Nationwide Register-based Cohort Study. 2025 Available at: https://dx.doi.org/10.2139/ssrn.5227321
16. UK Health Security Agency. Epidemiology of COVID-19 in England: January 2020 to December 2024 - Updated 29 May 2025. Available at: https://www.gov.uk/government/publications/epidemiology-of-covid-19-in-england/epidemiology-of-covid-19-in-england-january-2020-to-december-2024#vaccine-surveillance
17. Link-Gelles R, Chickery S, Webber A, al. e. Interim Estimates of 2024–2025 COVID-19 Vaccine Effectiveness Among Adults Aged ≥18 Years — VISION and IVY Networks, September 2024–January 2025 | MMWR Morbidity and Mortality Weekly Report (MMWR). 2025 Available at: http://dx.doi.org/10.15585/mmwr.mm7406a1
18. Appaneal HJ, Lopes VV, Puzniak L, Zasowski EJ, Jodar L, McLaughlin JM, et al. Early effectiveness of the BNT162b2 KP.2 vaccine against COVID-19 in the US Veterans Affairs Healthcare System. Nat Commun. 2025 Apr 29;16(1):4033. Available at: https://www.ncbi.nlm.nih.gov/pubmed/40301395
19. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022 Mar 5;399(10328):924-44. Available at: https://www.ncbi.nlm.nih.gov/pubmed/35202601
20. European Centre for Disease Prevention and Control. Acute respiratory infections in the EU/EEA: epidemiological update and current public health recommendations – winter 2024/2025. 2024. Available at: https://www.ecdc.europa.eu/en/news-events/acute-respiratory-infections-eueea-epidemiological-update-and-current-public-health-0