Introduction to the Annual Epidemiological Report
All disease surveillance reports in the Annual Report series draw on data retrieved from TESSy [1]
Note to the reader
This disease surveillance report presents an overview of the epidemiological situation for communicable diseases and related health issues that are under European Union (EU) and European Economic Area (EEA) surveillance [2],[3],[4],[5].
The annual epidemiological reports are available as a series of individual reports. The reports will be published as they become available.
Every year, the Member States electronically submit their surveillance data to The European Surveillance System (TESSy), with the last data sets usually received at the end of the following year.
ECDC’s annual surveillance reports are mainly intended for public health professionals and policymakers involved in disease prevention and control programmes and provide a wealth of epidemiological data to support decision-making at the national level.
Methods
Data sources: indicator-based disease surveillance
At least once a year, all EU Member States and three EEA countries (Iceland, Liechtenstein and Norway) send data from their surveillance systems to ECDC. All data relate to occurrences of cases of communicable diseases and health issues under mandatory EU-wide surveillance. Reports are sent in accordance with the case definitions established by the EU [6].
The information submitted to ECDC by the Member States is contained in a metadata set for each disease under surveillance. The metadata set includes case classifications (i.e. whether a case is confirmed or probable) based on the official case definitions by the European Commission. It also defines the information to be included with each case report.
Most data are submitted as anonymised individual case data, but some Member States report aggregate data for some diseases. Countries actively report zero cases for particular diseases.
Member States validate and upload their data with the European Surveillance System (TESSy), ECDC’s online system for the collection of surveillance data. Information specialists in the Member States transform the national data into a compatible format before uploading to TESSy. System reports generated by TESSy allow Member States to review and modify uploaded data. TESSy also performs automatic validity checks, while additional data validation is conducted by ECDC staff, in liaison with designated disease experts and epidemiologists in the Member States. The various reports are reviewed by Member States’ National Focal Points for Surveillance before publication. Final data corrections are then uploaded to TESSy.
For each disease under surveillance, the TESSy database holds a description of the key attributes of the national surveillance systems for that disease. This information is included in the reports to aid the interpretation of surveillance data for each reported disease. Member States are asked to verify and update this information each year.
Data sources: event-based surveillance
Event-based surveillance continuously monitors various information sources in order to rapidly detect and assess public health threats to the EU/EEA population. In addition, event-based surveillance is informed by direct reporting of relevant events from nominated Member State representatives through the Early Warning and Response System (EWRS) and from a wider community of national disease experts through the Epidemic Intelligence System (EPIS), depending on the type of event.
The analysis of monitored threats in this report covers the period from the activation of the system in June 2005 until the end of the reporting year; EWRS entries are covered from January 2005 to the end of the reporting year.
The two surveillance models complement each other and allow ECDC to achieve the agreed EU/EEA surveillance objectives. For example, indicator-based surveillance provides relevant background information for the assessment of threats detected by event-based surveillance, while event-based surveillance detects threats that are not detected by indicator-based surveillance because of reporting delays or because the threat is posed by a non-notifiable, emerging or previously unknown disease.
Data analysis
General principles
Whenever possible, analyses are based on confirmed cases in accordance with EU case definitions. For some diseases, some Member States do not report cases as ‘confirmed’ due to differences between national and EU case-definitions. For some diseases (e.g. tuberculosis, Legionnaires’ disease), confirmed cases are defined on a specific basis, which is described in the relevant sections of the reports.
For other diseases, restricting data analysis to only confirmed cases would result in severe underestimation of the true disease burden, hence both probable and confirmed cases are reported.
The variable ‘month’ used in the trend and seasonality analyses is based on the date that the Member State chooses as its preferred date for reporting. This could be either the date of disease onset, the date of diagnosis, the date of notification, or some other date at the country’s discretion.
Population data
Population data for the calculation of rates are obtained from Eurostat, the statistical office of the EU. Data for overall calculations are extracted from the Eurostat database ‘Demographic balance and crude rates’ (DEMO_PJAN). The population as of 1 January of each year is used. Totals per year and per country are available for all countries. For the calculation of age- and gender-specific rates, the data are aggregated into specific age groups. For the calculation of the notification rates for congenital syphilis and congenital toxoplasmosis, life births are used.
Presentation of analyses
Summary statistics for each disease are presented as a summary table by country. Supplementary figures are used to describe the overall epidemiology at the EU/EEA level. Most chapters include figures on the trend for reported confirmed cases, on age- and gender-specific rates, and on occurrence by month (‘seasonality’). Additional graphs, figures and maps are used to illustrate other important aspects of the disease epidemiology in the EU and EEA.
Summary table
This table presents an overview of the number and rates (including age-standardised rates) of cases reported by the Member States’ surveillance systems for the last five years. Depending on available data, the number of cases can refer to confirmed or total cases. The total number of reported cases for the last reporting year – regardless of case classification status – is also shown.
Notification rates are calculated per 100 000 persons: the number of reported confirmed/total cases, divided by the official Eurostat estimate of the population for that year, multiplied by 100 000. Countries that did not report disease data are excluded from the calculation of the overall European rate for that disease.
If the surveillance system coverage for a country is given, the calculation of the rates is adjusted depending on system coverage. Notification rates for congenital syphilis and congenital toxoplasmosis are calculated as cases per 100 000 live births.
The symbol ‘-’ is used when the indicator was not calculated. If no data were reported, the symbol ‘.’ is used. If countries report zero cases, the number zero is used.
Age-standardised rates (ASRs) are calculated to facilitate comparisons between countries by adjusting for differences with respect to certain underlying population characteristics such as age. ASRs are calculated when the EU/EEA rate exceeds 1 per 100 000 population and are given per 100 000 persons. ASRs are not calculated if relevant completeness (percentage of cases in the database with information on age) was less than 90%.
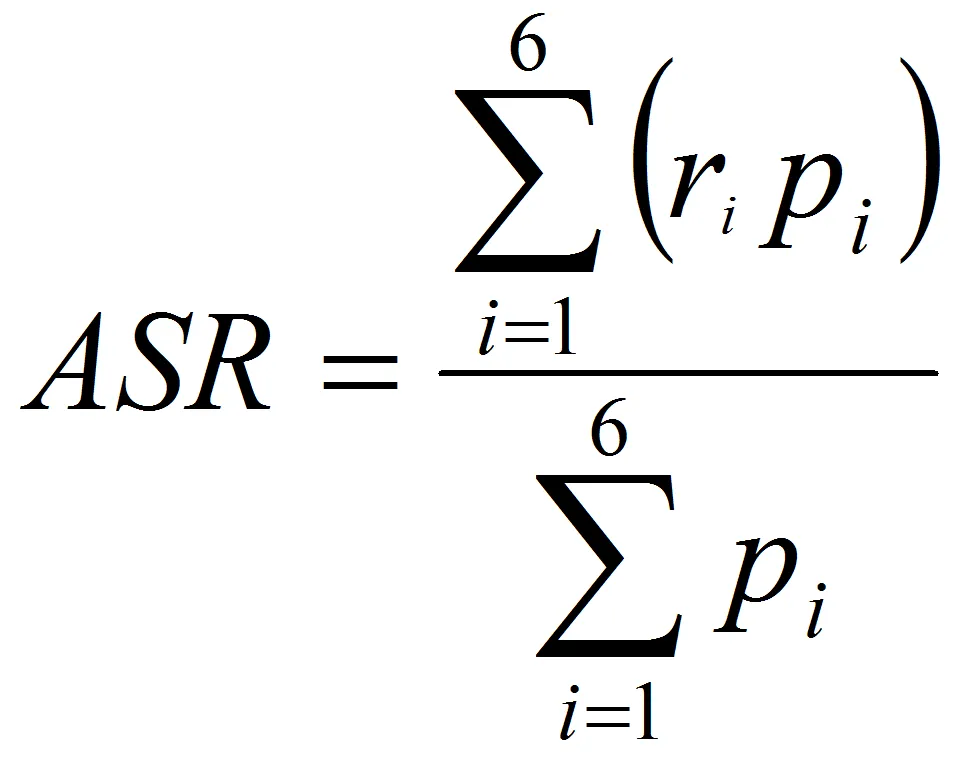
ASRs were calculated with this formula, see image on the right (direct method):
where ri is the specific rate for the age group i in the population studied, and pi is the population of age group i in the standard population.
The standard population considered in this report was based on the average population of the EU27 Member States for the period 2001–2010 (see table below). This standard population was defined to reflect the current age structure of Europe.
| Age group | Standard population |
|---|---|
| 0–4 | 25 506 062 |
| 5–14 | 54 043 285 |
| 15–24 | 62 075 051 |
| 25–44 | 143 411 393 |
| 45–64 | 124 427 054 |
| ≥65 | 81 889 316 |
| Total | 491 352 161 |
The summary table for each disease indicates the coverage of the national surveillance systems as follows:
Y= national coverage
N = known reported coverage in percent from a system that has no national coverage and where the size of the covered population is not reported
. = covered population is unknown
A = aggregate data
C = case-based data
Aspects of descriptive epidemiology at the EU/EEA level
Data are analysed as follows:
Trends in the reported number of cases. The number of confirmed (or total) cases for the EU/EEA by month is presented as a figure. All countries that consistently reported cases – or reported zero cases over the whole five-year period covered by the report – are included. If, in a calendar year, more than 50 percent of a country’s case records fail to include information on the month of reporting, the country is excluded from analysis. The trend figure also shows a centred 12-month moving average, illustrating the overall trend by smoothing seasonal and random variations.
Age- and gender-specific rates for confirmed cases. Age- and gender-specific rates for the EU/EEA Member States are presented and given per 100 000 persons. ECDC only calculates these rates if an 80% completeness threshold for age and gender data is met by individual countries and the EU/EEA as a whole.
For most of the diseases, cases were grouped into six age groups (0–4, 5–14, 15–24, 25–44, 45–64, ≥65). For some diseases, specific age groups are used.
Seasonal distribution of cases. If the reported occurrence of a disease varies markedly by month or season, a figure on seasonality is presented, showing the total number of confirmed cases reported for each month. These figures are then compared with the maximum, minimum and average number of cases observed for each month in the previous four years.
The analysis of seasonality uses the same completeness thresholds as described above.
Please note that for some diseases the numbers reported are too small to conduct a meaningful analysis.
Data protection
Member State data received by TESSy are subject to Regulation (EC) No 45/2001 of the European Parliament and of the Council of 18 December 2000, providing for ‘the protection of individuals with regard to the processing of personal data by the Community institutions and bodies, and on the free movement of such data.’
High standards of data protection consistent with these requirements are applied and supervised by the ECDC Data Protection Officer. ECDC data protection arrangements are also under the review of the European Data Protection Supervisor.
Data are made available on request to other European agencies, institutions and approved researchers, under procedures in accordance with the above requirements and by approval of the ECDC Management Board.
[1] The European Surveillance System (TESSy) is a system for the collection, analysis and dissemination of data on communicable diseases. EU Member States and EEA countries contribute to the system by uploading their infectious disease surveillance data at regular intervals.
[2] 2000/96/EC: Commission Decision of 22 December 1999 on the communicable diseases to be progressively covered by the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Official Journal, OJ L 28, 03.02.2000, p. 50–53.
[3] 2003/534/EC: Commission Decision of 17 July 2003 amending Decision No 2119/98/EC of the European Parliament and of the Council and Decision 2000/96/EC as regards communicable diseases listed in those decisions and amending Decision 2002/253/EC as regards the case definitions for communicable diseases. Official Journal, OJ L 184, 23.07.2003, p. 35–39.
[4] 2007/875/EC: Commission Decision of 18 December 2007 amending Decision No 2119/98/EC of the European Parliament and of the Council and Decision 2000/96/EC as regards communicable diseases listed in those decisions. Official Journal, OJ L 344, 28.12.2007, p. 48–49.
[5] Commission Decision 2119/98/EC of the Parliament and of the Council of 24 September 1998 setting up a network for the epidemiological surveillance and control of communicable diseases in the Community. Official Journal, OJ L 268, 03/10/1998 p. 1-7.
[6] 2002/253/EC: Commission Decision of 19 March 2002 laying down case definitions for reporting communicable diseases to the Community network under Decision No 2119/98/EC of the European Parliament and of the Council. Official Journal, OJ L 86, 03.04.2002, p. 44–62.
