EU Laboratory Capability Monitoring System (EULabCap): Report on 2016 survey of EU/EEA country capabilities and capacities
Executive Summary
Background
ECDC aims to foster and reinforce the EU public health microbiology system to provide timely and reliable information for infectious threat detection, the assessment of such threats, and their surveillance at the Member State and EU levels, thus ensuring the effective prevention and early control of infectious diseases [4]. To ascertain how well this is delivered, ECDC developed, in close collaboration with national microbiology focal points from all European Union/European Economic Area (EU/EEA) countries and the ECDC Advisory Forum, the EULabCap survey methodology for monitoring. The EULabCap survey assesses, on an annual basis, key public health microbiology capabilities and capacities for EU surveillance and epidemic preparedness. The EULabCap results help policymakers at all levels identify possible areas for action and evaluate the impact of capacity strengthening activities and healthsystem reform.
This fourth EULabCap report presents EU/EEA laboratory capabilities and capacities for 2016 and compares them with previous survey results [6-8].
Methods
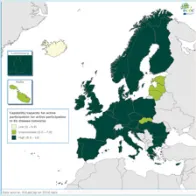
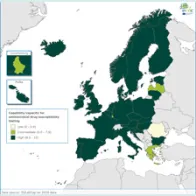
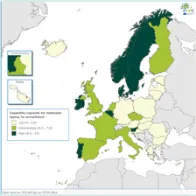
The EULabCap monitoring tool combines 60 indicators to assess the capability and capacity of microbiology laboratories to provide essential public health functions, as defined in EU policies and action plans, international health regulations, and technical standards. The EULabCap indicators comprise 24 structure and 36 process indicators. They are grouped into 12 targets distributed across three dimensions: primary diagnostic testing, national microbiology reference laboratory services, and laboratory-based surveillance and epidemic response support. Each indicator can be scored at three levels: low, intermediate or high capability/capacity. Aggregated indices were calculated for each target and dimension as the average of component indicator scores; all index values are displayed on a scale of 0–10. In 2016, two indicators were not applicable, and two indicators were replaced by new ones to reflect new and updated EU standards.
A mixed method was used for data collection and scoring, which took place from October to December 2017. To minimise the data reporting burden for the Member States, ECDC retrieved information for 18 indicators from TESSy datasets (The European Surveillance System) and EU disease network reports. For the remaining 40 indicators, the national microbiology focal points (NMFP) used a questionnaire to collect information from their country. The data collected for 2016 were validated by the NMFP in December 2017. As soon as validated data became available, country profile reports and benchmarking results were shared with the NMFP so that they could inform the national stakeholders about key results and areas that required attention. Maps illustrating the country scores (EULabCap index levels) were published on the ECDC Web portal.
In January–March 2018, an NMFP feedback survey was conducted on the dissemination and use of the EULabCap reports for 2014 and 2015 to develop measures for capacity strengthening at the national level.
Results
The country response rate to the 2016 survey was 100%. Data were provided for 97% of the applicable indicators (range per country, 90–100% complete data available).
The average EULabCap 2016 index for all EU/EEA countries was 7.5 on a scale of 0–10, as compared to 7.5 in 2015, 7.3 in 2014, and 6.9 in 2013. In 2016, individual EULabCap indices per country ranged from 5.6 to 9.6 as compared to 4.7 to 9.2 in 2013, indicating that differences between national systems gradually decreased over the period 2013–2016. Ten countries improved their EULabCap index; five climbed from low to fair, and another five countries went from fair to high.
Average EULabCap scores varied among the EULabCap public health targets, with improvement found in many areas, but also consistently low scores in two areas:
- Strong overall EU/EEA capacities since 2013: antimicrobial drug susceptibility testing; antimicrobial drug resistance monitoring; laboratory collaboration within national and EU surveillance networks; provision and regulation of NRL microbiology services; and reference diagnostic confirmation for EU notifiable diseases;
- Improved capabilities across Europe in 2016: provision and regulation of clinical and reference microbiology services, diagnostic testing guidance, contribution of reference laboratories to detection and response to emerging diseases and multi-drug resistance threats;
- Persistent low capacity for many countries in 2016: utilisation of diagnostic testing and molecular typing data reporting for EU surveillance.
Not all EU/EEA Member States have reached sufficient levels of laboratory capability and capacity across all targets to conduct effective public health surveillance and provide an adequate level of disease threat response. In 2016, as in 2015, 19 countries fulfilled sufficient (fair to high) capacity levels for at least 10 of 12 EULabCap targets. Eight of these countries scored sufficient capacity levels for all targets.
The NMFP of 27 EU/EEA countries participated in a survey on national dissemination and use of EULabCap reports. The EU LabCap country reports based on 2013–16 data were disseminated by NMFP to stakeholders in all responding countries; they were considered useful in 23 countries as a tool for advising national authorities. Overall, the EULabCap country reports were mainly discussed with microbiologists and epidemiologists at the national level; in 17 countries the reports were communicated also to decision makers or senior management.
In 24 countries, follow-up actions were undertaken between August 2015 and March 2018 to address areas that required attention while three countries reported no follow-up activities. National capacity strengthening efforts focused on the following five areas: quality of reporting of microbiological surveillance data to ECDC, regulation of national reference laboratory services, transition to WGS for typing, involvement of reference laboratory in outbreak investigations, and development of national diagnostic testing guidance.
Conclusions
The high response rate to the EULabCap surveys highlights the continued commitment of EU/EEA countries to this health system benchmarking process. It also enables a robust assessment of collective EU/EEA and country-level laboratory system capacity. The results of this fourth annual survey confirm that the EU/EEA, with an aggregated index score of 7.5/10 for 2016, can rely on microbiology services that are already fairly strong and contribute substantially to public health capabilities that are continuously improving.
Overall, public health microbiology services in the EU/EEA meet most key requirements for communicable disease surveillance and response. However, not all EU/EEA Member States have yet reached sufficient levels of laboratory capability and capacity across all targets assessed by EULabCap in order to deliver effective public health surveillance and threat response. ‘Sufficient microbiology capacity’ (defined as intermediate or high capacity for at least 10 of 12 EULabCap targets) was achieved by 19 of the 30 EU/EEA Member States in 2015–16.
Steady increases in the average EULabCap indices of a substantial number of countries over the past four years suggest that public health microbiology shortcomings are being addressed. Narrowing variation in the EULabCap index between countries over the past years indicates technical convergence and progress toward a more equitable balance of laboratory capacities among Member States.
The survey results can assist countries in focussing their efforts to achieve a level of ‘sufficient microbiology capacity’. EULabCap monitoring provides detailed EU/EEA benchmarking information for national competent bodies and policymakers at the national level. It is noteworthy that results from the 2018 feedback survey indicated that the annual EULabCap reports were disseminated to stakeholders in all countries. EULabCap reports were also considered useful for advising national authorities in the vast majority of the participant countries where relevant system capacity strengthening actions were carried out over the last years.
Gaps and inefficiencies still to be addressed in some countries include the development of wider clinical guidance for upgrading to genomic methods for the detection and characterisation of epidemic agents, guidance on the adequate utilisation of diagnostic tests, and enhanced digital connections between laboratory information and public health monitoring and early warning systems at national and EU levels. These gaps were reviewed by ECDC, the competent bodies in the Member States, the European Commission, and several international partners in 2017 to inform the ECDC microbiology priorities and support activities for 2018–22.
2016_EULabCap report
English (8.68 MB - PDF)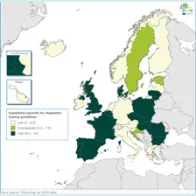

Share this page