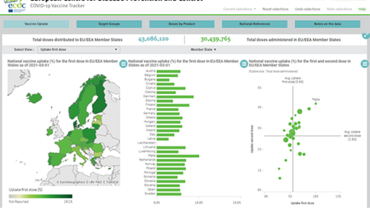
COVID-19 vaccination and prioritisation strategies in the EU/EEA
This document builds on a previously published ECDC report, ‘Key aspects regarding the introduction and prioritisation of COVID-19 vaccination in the EU/EEA and the UK’. By using mathematical modelling, this document provides EU/EEA countries with information on factors that may affect the choice of COVID-19 vaccination strategies, according to different target groups and based on scenarios of hypothetical vaccine characteristics.
Executive Summary
Key facts
- Prioritisation of COVID-19 vaccination should take into account several dimensions and needs to be contextualised.
- The choice of optimal vaccination strategy depends on the objective, e.g. reducing mortality, saving life years or reducing pressure on the healthcare system.
- The optimal prioritisation also depends on the characteristics of the vaccine, in particular its efficacy against infection and therefore onward transmission.
- If a vaccine does not protect against transmission, the most effective and efficient approach is to prioritise the vaccination of those groups at highest risk of severe disease and death.
- Substantial reductions in mortality and pressure on the healthcare system could be achieved by the direct protection of high-risk groups, even if viral transmission is ongoing within the population.
- Vaccination of healthcare workers is beneficial since it improves the resilience of the healthcare system. The benefit would be heightened if the vaccine were effective against infection, and therefore transmission, since it would offer indirect protection to patients, residents of long-term care facilities and other high-risk individuals.
- Although vaccinating adults aged 18-59 years is not the most effective or efficient strategy when vaccine supply is limited, consideration should be given to specific groups or settings that may have a disproportionate risk of exposure.
- Given the many unknowns in relation to COVID-19 vaccines’ characteristics, deployment, supply, and uptake, and to future appearance of vaccine escape variants, non-pharmaceutical interventions should continue to be applied, as recommended by public health authorities, in the initial months following the introduction of COVID-19 vaccination.
- Vaccination strategies will need to be adaptable over time to unfolding events taking into account the emerging evidence.
Download

Read more
First COVID-19 vaccine authorised for use in the European Union
ECDC welcomes the authorisation of the first vaccine against COVID-19 in EU/EEA. We have been looking forward to this moment for a long time.
On 21 December, EMA recommended granting a conditional marketing authorisation for the vaccine Comirnaty, developed by BioNTech and Pfizer, to prevent coronavirus disease 2019 (COVID-19) in people from 16 years of age. On the same day, the European Commission has granted market authorisation for EU-wide use.
ECDC releases COVID-19 vaccination rollout strategies for EU/EEA
On 22 December ECDC released the technical report titled ‘COVID-19 vaccination and prioritisation strategies in the EU/EEA’, following the European Medicines Agency (EMA) recommendation for authorisation of the vaccine Comirnaty, developed by BioNTech and Pfizer.
Key aspects regarding the introduction and prioritisation of COVID-19 vaccination in the EU/EEA and the UK
This document provides an overview of the key aspects related to the initial phases following the introduction of one or more COVID-19 vaccines in the European Union and European Economic Area (EU/EEA) and the United Kingdom (UK). The aim is to support but not define EU policy on COVID-19 vaccination.