Epidemiological update: Echovirus 11 infections in neonates
ECDC is monitoring the situation closely and encourages Member States to report any case fitting the case definition through EpiPulse (Item ID 2023-EIP-00026).
Disease background
Enteroviruses (EV) are a group of viruses that usually cause self-limited to mild illness. In certain populations, such as neonates, infection by specific serotypes of EV can cause severe illness. The most relevant EV subspecies in neonatal infections include Coxsackievirus B and Echovirus, including multiple distinct serotypes. Clinical manifestations of EV infection may range from asymptomatic, acute febrile illness to life-threatening disseminated disease. Echovirus 11 (E11) infection in neonates may be associated with severe clinical features, such as sepsis, myocarditis, and meningitis. The most characteristic clinical syndrome in neonates infected with E11 is fulminant hepatitis presenting with restlessness, vomiting, jaundice, growth restriction, hepatomegaly, electrolyte imbalance, metabolic acidosis, ascites, peripheral oedema, hypoglycaemia, and bleeding diathesis.
EVs are predominately transmitted via faecal-oral and respiratory routes. For previously reported cases of E11 infection in neonates, suspected modes of transmission included vertical transmission (prenatal transplacental or during childbirth), postnatal human-to-human contact, as well as being spread through nurseries by caregivers and neonatal intensive care units by healthcare workers. Transmission by breastfeeding has also been reported as possible. Several outbreaks due to E11 infection in neonates, including those that were healthcare-acquired, have been previously reported [1-6]. Some of the outbreaks are reported to have occurred in the context of community circulation of E11.
Data on non-polio enterovirus are not currently collected on a European level, but country reports and studies give insights into circulation of enteroviruses. A study published in 2002 focusing on non-polio enterovirus infections in neonatal intensive care units in the Netherlands estimated the incidence at 26 per 100 000 neonates (age ≤30 days) [7]. A retrospective surveillance from 2015–2017 study among EU/EEA Member States investigated seasonality, clinical symptoms age-group distribution and genotype distribution of three enteroviruses [8]. An investigation of enterovirus circulation in EU/EEA countries between 2015 and 2017 highlighted the wide circulation of non-polio enteroviruses in Europe, mostly affecting young children, with E11 among the five most commonly notified from children aged under three months [8]. For previously reported cases in neonates, infection and death outcomes have been more frequently associated with E11 when compared with other EVs in the same population.
Epidemiological update
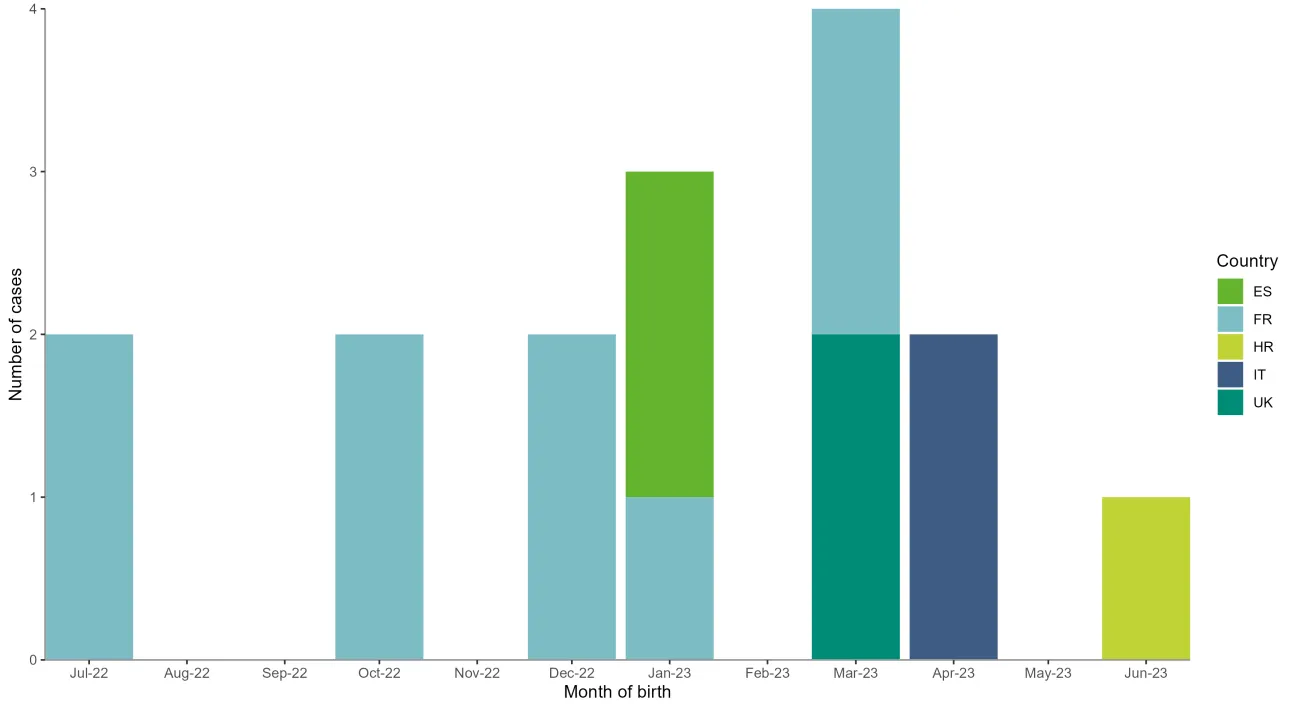
Since 2022, and as of 17 July 2023, 19 neonates with severe Echovirus 11 (E11) infection have been reported in the EU/EEA, by France, Croatia, Sweden, Spain, and Italy, and nine of these neonates have died (Figure 1). The UK has also reported two fatal E11 neonatal cases. Austria, Belgium, Denmark, the Netherlands, Norway, and Portugal have not observed an increase of E11 infections associated with severe neonatal cases compared to previous years.
Since July 2022, nine neonates have been diagnosed in France with severe sepsis, complicated by hepatic failure, and neurological or myocardial involvement due to infection with E11. All cases were male: four pairs of premature twins, and a full-term singleton. All presented clinical signs at three to six days old, and five of the nine neonates were born with low birth weight. Maternal clinical symptoms, such as fever and gastrointestinal signs, were reported in four of five mothers during the three days before or on delivery. Seven of the neonates died [9].
Two cases of fulminant hepatitis linked with E11 infection in late pre-term twin brothers were reported in June by Italy. The mother presented with a single episode of fever at 35 weeks and two days of gestational age. The neonates have been transferred to a neonatal intensive care unit (NICU). The phylogenetic and molecular analysis concluded that the Italian E11 strains clustered with French strains collected in 2023 [10]. Italy has reported a third E11 case in a neonate, who was admitted to NICU.
Public health authorities in Spain have reported two cases of severe E11 infection. These cases were preterm twins born in January 2023. Both cases were admitted to the NICU after birth with one recorded death and a diagnosis of severe enterovirus infection with probable vertical transmission, while the second case was discharged from the hospital without sequelae. [11]
Public health authorities in the UK have notified that an E11 neonatal sepsis event with a fatal outcome occurred soon after birth in a pair of twins in March 2023.
Since January 2022, and as of June 2023, four cases of E11 viral meningoencephalitis have been notified in Sweden among infants, two of whom were under 10 days old and two between 25 and 35 days old.
According to media reports quoting health authorities, two clusters of enterovirus disease in neonates have been reported from two different hospitals in Croatia. Typing efforts are ongoing and to date infection with E11 has been confirmed in only one of the cases. Symptoms include meningoencephalitis and myocarditis.
Further cases of E11 infection have been reported in 2022 and 2023 in neonates, infants, and older children, without full information of the clinical manifestations or outcomes. However, Austria, Belgium, Denmark, the Netherlands, Norway, and Portugal have not observed an increase of E11 infections associated with severe neonatal cases.
Figure 1. Distribution of confirmed and probable cases of severe neonatal Echovirus 11 infection in the EU/EEA and UK as of June 2023
Case definition used in the EU/EEA
Data on non-polio enterovirus infections and their disease burden in the EU/EEA are not currently collected at European level. Non-polio enterovirus infections are not notifiable in most EU/EEA countries and with substantial variation in surveillance approaches between countries [8,12].
While it is difficult to interpret observations on severe neonatal Echovirus 11 (E11) infection in the absence of robust historical data, Member States should consider raising awareness of the importance of including E11 infection in the differential diagnosis of hepatitis, sepsis, myocarditis or pericarditis, neurological and severe respiratory illness of neonates in order to identify cases and initiate appropriate precautions, as well as to provide more robust epidemiological information. To investigate the current situation in attempt to better characterise the risk for neonates, ECDC asks Member States to provide information, through EpiPulse (Item ID 2023-EIP-00026) on cases following the below case definitions:
- Confirmed case: Neonates (<28 days) admitted to NICU with laboratory-confirmed diagnosis of echovirus 11 lineage 1* notified since 1 January 2022.
- Probable case: Neonates (<28 days) admitted to NICU with laboratory-confirmed diagnosis of echovirus 11 notified since 1 January 2022,
- Suspect case: Neonates (<28 days) admitted to NICU with laboratory-confirmed diagnosis of other non-polio enterovirus notified since 1 January 2022.
*Lineage 1 as outlined by Grapin et al., 2023 molecular characterisation of the new E11 lineage [9].
Data collection
ECDC is particularly interested in the following information in a case-based format where possible:
- Basic demographic characteristics and perinatal history;
- Clinical course, treatment, and outcome;
- Potential of vertical transmission and healthcare-associated infection;
- Laboratory testing of the case and contacts (mother/other family members, HCW), including sample type collected and other pathogens tested and/or detected;
- Sequencing and molecular typing of echovirus. While whole genome sequencing is preferred, VP1 sequencing data are also of interest; VP1 sequences should span at least the positions 2568-2800 (numbered as in the Bastianni prototype sequence, AF311938.
Furthermore, if available, Member States should report whether there has been an increased background circulation of enteroviruses in the general population at the time cases were identified.
Results from laboratory testing should be reported to local and national public health authorities. The nominated national authorities should then report relevant data to EpiPulse (Item ID 2023-EIP-00026), ECDC’s secure reporting platform for nominated users of national public health authorities.
ECDC recommends sharing genome sequence data in the public domain (ENA, GenBank) or via EpiPulse to allow easy access for all international stakeholders and sharing with ECDC for inclusion in multi-country analysis. ECDC will incorporate new sequencing data into phylogenetic analysis as additional data become available. The retrospective aspect of this data collection (from January 2022) should facilitate tracing the emergence of a new divergent lineage.
Testing guidance
Most EU/EEA Member States perform laboratory-based national surveillance for non-polio Enterovirus detections with typing competency at the national reference laboratory [12]. In the context of this situation, ECDC recommends testing for E11 in all NICU admissions with one or more of the following clinical manifestations that cannot be explained by other causes: sepsis, myocarditis or pericarditis, hepatitis, encephalitis, and pneumonia complicated with respiratory failure.
The specimen type needed for diagnostics depends on the clinical manifestation and time since onset. Sampling should be performed accordingly and as soon as possible after symptom onset/NICU admission. Specimen types that may be collected include faecal, cerebrospinal fluid, respiratory specimen/nasopharyngeal swab, blood, conjunctival swab, biopsy specimen, and urine [13]. Vertical transmission appears a likely route of infection for several cases reported in this event and testing of the mothers or other family members as well as collecting their recent clinical history in terms of possible enterovirus infection is of importance.
For screening of enterovirus infection RT-PCR assays for enteroviruses, or a diagnostic panel including enteroviruses should be used. Any NICU admission with compatible symptoms testing positive for enterovirus infection should undergo further testing to determine the presence of E11 by corresponding reference laboratories. For typing to E11, sequencing of the complete genome or partial genome including the VP1 region from the clinical sample should be performed to confirm the presence of E11 and enable phylogenetic analysis.
Treatment and prevention
No enterovirus vaccine other than polio vaccine is licensed in the EU/EEA, and there is currently no antiviral treatment for EV infections. However, efforts are being made to develop broad spectrum antivirals [14-16], and small studies and case-reports indicate the potential benefit of intravenous immunoglobulin [16,17]. Early diagnosis and the administration of treatments following national guidelines are important to minimise the impact of E11 infection. A recent E11 outbreak in neonates identified several risk factors associated with haemorrhage-hepatitis syndrome compared to mild disease, including prematurity, low birth weight, premature rupture of fetal membrane, and total or partial parenteral nutrition prior to onset of disease [18]. Strict hygienic practices, such as frequent hand-washing, avoidance of shared utensils, bottles, or glasses, and disinfection of contaminated surfaces (e.g. with diluted bleach solution) are recommended to prevent the spread of E11 from person to person.
Assessment
Based on the available information, ECDC assesses the likelihood of infection with E11 among the neonatal population to be very low, with a high level of uncertainty. The impact of infection is estimated to be moderate, with a high level of uncertainty. Therefore, the overall public health risk for the neonatal population of the EU/EEA is currently estimated to be low. More data are needed to assess whether the new divergent E11 lineage is associated with more severe disease. ECDC will reassess the risk once more information becomes available.
Actions and plans
ECDC is monitoring the situation closely and encourages Member States to report any case fitting the case definition through established means of communicating in EpiPulse (Item ID 2023-EIP-00026). In addition, ECDC is collaborating on a retrospective study that will be carried out by the European Non-Polio Enterovirus Network (ENPEN), aiming at describing the EV circulation among neonates and infants in Europe between 2018 and 2023. Participation in this study is distinct from any data collected on EWRS or EpiPulse, and specific invites for it will be circulated shortly.
References
- Berkovich S, Kibrick S. ECHO II outbreak in newborn infants and mothers. Pediatrics. 1964 Apr;33:534-40.
- Cramblett HG, Haynes RE, Azimi PH, Hilty MD, Wilder MH. Nosocomial infection with Echovirus type II in handicapped and premature infants. Pediatrics. 1973 Apr;51(4):603-7.
- Piraino FF, Sedmak G, Raab K. Echovirus 11 infections of newborns with mortality during the 1979 enterovirus season in Milwaukee, Wis. Public health reports (Washington, DC : 1974). 1982 Jul-Aug;97(4):346-53.
- Chow CB, Tse HH, Chan KY, Tam A, Ho LC, Ho WY, et al. Outbreak of Echo Virus Type 11 Infection in Newborn Infants in a Maternity Ward: Clinical Presentation. Journal of Tropical Pediatrics. 1987;33(6):305-8. Available at: https://doi.org/10.1093/tropej/33.6.305
- Bina Rai S, Wan Mansor H, Vasantha T, Norizah I, Chua KB. An outbreak of echovirus 11 amongst neonates in a confinement home in Penang, Malaysia. The Medical journal of Malaysia. 2007 Aug;62(3):223-6.
- Ho S-Y, Chiu C-H, Huang Y-C, Chen C-J, Lien R, Chu S-M, et al. Investigation and successful control of an echovirus 11 outbreak in neonatal intensive care units. Pediatrics & Neonatology. 2020 2020/04/01/;61(2):180-7. Available at: https://www.sciencedirect.com/science/article/pii/S1875957219305212
- Verboon-Maciolek MA, Krediet TG, van Loon AM, Kaan J, Galama JM, Gerards LJ, et al. Epidemiological survey of neonatal non-polio enterovirus infection in the Netherlands. Journal of medical virology. 2002 Feb;66(2):241-5.
- Bubba L, Broberg EK, Jasir A, Simmonds P, Harvala H, Redlberger-Fritz M, et al. Circulation of non-polio enteroviruses in 24 EU and EEA countries between 2015 and 2017: a retrospective surveillance study. The Lancet Infectious Diseases. 2020 2020/03/01/;20(3):350-61. Available at: https://www.sciencedirect.com/science/article/pii/S1473309919305663
- Grapin M, Mirand A, Pinquier D, Basset A, Bendavid M, Bisseux M, et al. Severe and fatal neonatal infections linked to a new variant of echovirus 11, France, July 2022 to April 2023. Euro Surveill. 2023 Jun;28(22)
- Piralla A, Borghesi A, Di Comite A, Giardina F, Ferrari G, Zanette S, et al. Fulminant echovirus 11 hepatitis in male non-identical twins in northern Italy, April 2023. Eurosurveillance. 2023;28(24):2300289. Available at: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2023.28.24.2300289
- World Health Organization. Enterovirus-Echovirus 11 Infection in the European Region. Disease Outbreak News. 2023 07 July 2023 Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON474
- Harvala H, Jasir A, Penttinen P, Pastore Celentano L, Greco D, Broberg E. Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area, 2016. Euro Surveill. 2017 Nov;22(45)
- Harvala H, Broberg E, Benschop K, Berginc N, Ladhani S, Susi P, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. Journal of Clinical Virology. 2018 2018/04/01/;101:11-7. Available at: https://www.sciencedirect.com/science/article/pii/S1386653218300088
- Benschop KS, van der Avoort HG, Duizer E, Koopmans MP. Antivirals against enteroviruses: a critical review from a public-health perspective. Antivir Ther. 2015;20(2):121-30.
- Sun L, Meijer A, Froeyen M, Zhang L, Thibaut HJ, Baggen J, et al. Antiviral Activity of Broad-Spectrum and Enterovirus-Specific Inhibitors against Clinical Isolates of Enterovirus D68. Antimicrob Agents Chemother. 2015 2015/12//;59(12):7782-5. Available at: http://europepmc.org/abstract/MED/2636997
https://doi.org/10.1128/AAC.01375-1
https://europepmc.org/articles/PMC464916
https://europepmc.org/articles/PMC4649165?pdf=render - Chuang Y-Y, Huang Y-C. Enteroviral infection in neonates. Journal of Microbiology, Immunology and Infection. 2019 2019/12/01/;52(6):851-7. Available at: https://www.sciencedirect.com/science/article/pii/S1684118219301549
- Chetty K, Cheng I, Kaliakatsos M, Gonzalez-Granado LI, Klapsa D, Martin J, et al. Case report: Novel treatment regimen for enterovirus encephalitis in SCID. Frontiers in immunology. 2022;13:930031.
- Wang P, Xu Y, Liu M, Li H, Wang H, Liu Y, et al. Risk factors and early markers for echovirus type 11 associated haemorrhage-hepatitis syndrome in neonates, a retrospective cohort study. Frontiers in pediatrics. 2023;11:1063558.
Read more
Share this page