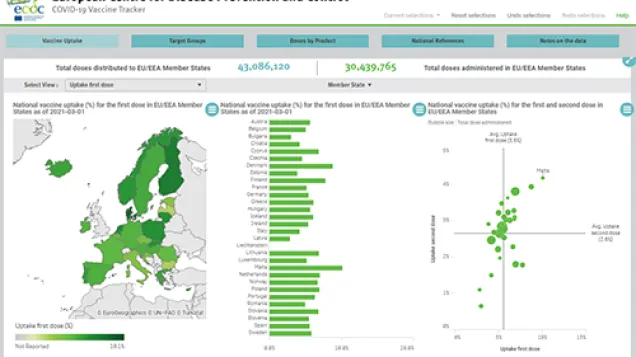
ECDC and EMA update on COVID-19
With the increasing circulation of the Delta variant of SARS-CoV-2 in EU/EEA countries, the European Medicines Agency (EMA) and the European Centre for Disease Prevention and Control (ECDC) strongly encourage those who are eligible for vaccination but have not yet been vaccinated to start and complete the recommended COVID-19 vaccination schedule in a timely manner.
Full vaccination with any of the EU/EEA-approved vaccines offers a high level of protection against severe disease and death caused by SARS-CoV-2, including variants, such as Delta. The highest level of protection is achieved after enough time (seven to fourteen days) has passed from the day of the last vaccine dose.
Vaccination is also important for protecting those at highest risk of severe disease and hospitalisation, reducing the spread of the virus, and preventing the emergence of new variants of concern.
Mike Catchpole, ECDC Chief Scientist said:
“While the available vaccines are highly effective in protecting people against severe COVID-19, until higher proportions of the population are immunised, the risk is not beyond us. We are now witnessing an increasing number of COVID-19 cases across the EU/EEA and vaccines remain the best available option to avoid an increase in severe disease and death.”
As vaccination campaigns gather pace across the EU/EEA, it may be advisable in some cases to consider reducing the interval between first and second doses, within the authorised limits, particularly for people at risk of severe COVID-19 who have not completed the recommended vaccine schedule.
Infections in vaccinated people do not mean that vaccines do not work
Although the effectiveness of all COVID-19 vaccines authorised in the EU/EEA is very high, no vaccine is 100% effective. This means that a limited number of SARS-CoV-2 infections among persons that have completed the recommended vaccination schedule (i.e. ‘breakthrough infections’) are expected. However, when infections do occur, vaccines can prevent severe disease to a large extent, and greatly reduce the number of people in hospital due to COVID-19.
Fergus Sweeney, EMA’s Head of Clinical Studies and Manufacturing said:
‘'These COVID-19 vaccines are very effective. However, as long as the virus continues to circulate, we will continue to see breakthrough infections in vaccinated people. This does not mean that the vaccines are not working. Vaccinated people are far better protected against severe COVID-19 than unvaccinated people, and we should all endeavour to be fully vaccinated at the earliest opportunity.”
EMA and ECDC recommend full COVID-19 vaccination for all eligible citizens [1]. Until more people are fully vaccinated, and while SARS-CoV-2 is still spreading, everyone should adhere to national regulations and continue to take measures such as wearing masks and respecting social distancing, even those individuals who have received a complete vaccination schedule.
EMA and ECDC remain committed to working closely with other EU bodies and national agencies to gather, produce and share the best scientific data to help Member States protect public health in the context of their national situations.
More information is available from our websites:
- European Centre for Disease Prevention and Control: COVID-19
- European Medicines Agency: Coronavirus disease (COVID-19)
- European Vaccination Information Portal
- 14 July EMA ECDC joint statement
Notes
[1] The product information for each authorised COVID-19 vaccine describes the full vaccination schedule. For Comirnaty, Spikevax and Vaxzevria it involves two doses taken within a certain timeframe. For COVID-19 Vaccine Janssen, only one dose is required.
Contact our press officers
ECDC press office
Tel. +46 (0)8 586 01 678
Email: press ecdc [dot] europa [dot] eu (press[at]ecdc[dot]europa[dot]eu)
ecdc [dot] europa [dot] eu (press[at]ecdc[dot]europa[dot]eu)
EMA press office
Tel. +31 (0)88 781 8427
E-mail: press ema [dot] europa [dot] eu (press[at]ema[dot]europa[dot]eu)
ema [dot] europa [dot] eu (press[at]ema[dot]europa[dot]eu)
Latest on COVID-19
Share this page