First COVID-19 vaccine authorised for use in the European Union
ECDC welcomes the authorisation of the first vaccine against COVID-19 in EU/EEA. We have been looking forward to this moment for a long time.
On 21 December, EMA recommended granting a conditional marketing authorisation for the vaccine Comirnaty, developed by BioNTech and Pfizer, to prevent coronavirus disease 2019 (COVID-19) in people from 16 years of age. On the same day, the European Commission has granted market authorisation for EU-wide use.
On this occasion, ECDC Director Andrea Ammon said: “Availability of safe and efficacious vaccines – a most powerful tool in ensuring public health – is an important milestone in the course of this pandemic. It is also clear that vaccines will do their work alongside with other measures that keep the spread of the infection under control. It will not happen at once and, for the time being, we have to remain patient and vigilant to protect each other by physical distancing, observing good hand and respiratory hygiene and use of face masks where necessary. Taken the current epidemiological situation in many countries, restrictions will have to continue well into 2021 until we see the impact of vaccination.”
COVID-19 vaccines will first reach those that are most in need of protection. National authorities have defined and identified priority groups for vaccination – they include healthcare workers, the elderly and the vulnerable. With this, the goal is first to protect those most at risk from severe disease, as well as slow down the ongoing pandemic and reduce the enormous burden that health systems are currently experiencing. As the situation evolves and we will learn more about the protective characteristics of various COVID-19 vaccines, vaccination strategies and objectives will be adapted and optimised. Once the pandemic becomes more manageable, societies will be able to take the big step of easing up on current restrictions.
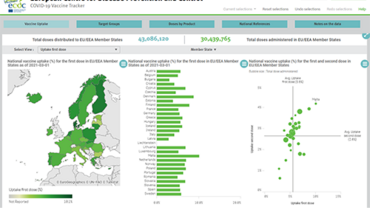
ECDC, together with EMA, has an important role to play in continually monitoring the effectiveness, safety and impact of the vaccines, to ensure that vaccines perform as expected and to inform if vaccination strategies need to be adapted. ECDC will continue periodically collect and report information from EU Member States on their vaccination deployment plans and strategies and will support decision-making by providing insights from modelling work.
ECDC will continue to monitor the course of the pandemic, issuing risk assessments and technical guidance as it has tirelessly done throughout this year. In this festive season, we would like to remind about the importance of protecting each other by avoiding non-essential travel, spending the festive season with as few people as possible and observing physical distancing, good hand and respiratory hygiene and wearing face masks as appropriate.
Links
- EMA recommendation: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
- Facts about COVID-19 vaccines on European Vaccination Information Portal: https://vaccination-info.eu/en/covid-19/covid-19-vaccines
- Announcement on market authorisation by the European Commission: Safe COVID-19 vaccines for Europeans | European Commission (europa.eu)